ScreenCell® simplifie l’isolement et l’analyse des cellules tumorales circulantes pour accélérer la recherche et améliorer la prise en charge des patients atteints de cancer.
Nous transformons la recherche sur les cellules tumorales circulantes en solution concrète pour améliorer les soins aux patients atteints de cancer.
Découvrez la gamme complète de kits ScreenCell®, chacun spécifiquement conçu pour répondre aux divers besoins des applications de biopsie liquide.
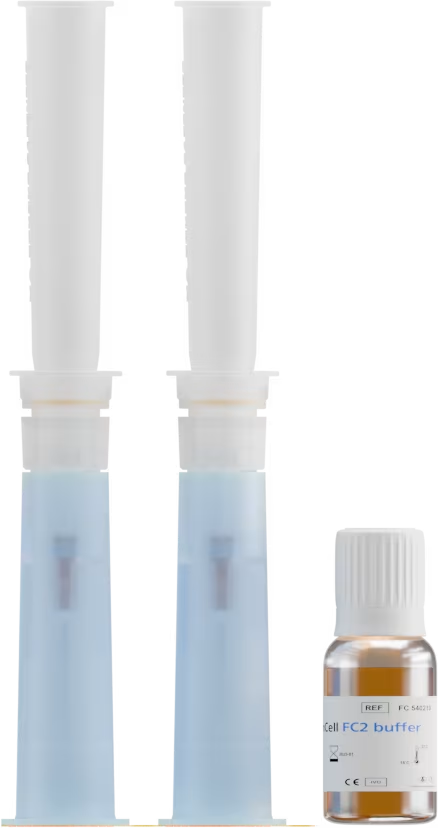
Échantillon de sang
< 4 heures
Cellules tumorales fixées
sur le support d’isolement

Échantillon de sang
< 3 jours
Cellules tumorales fixées
sur le support d’isolement
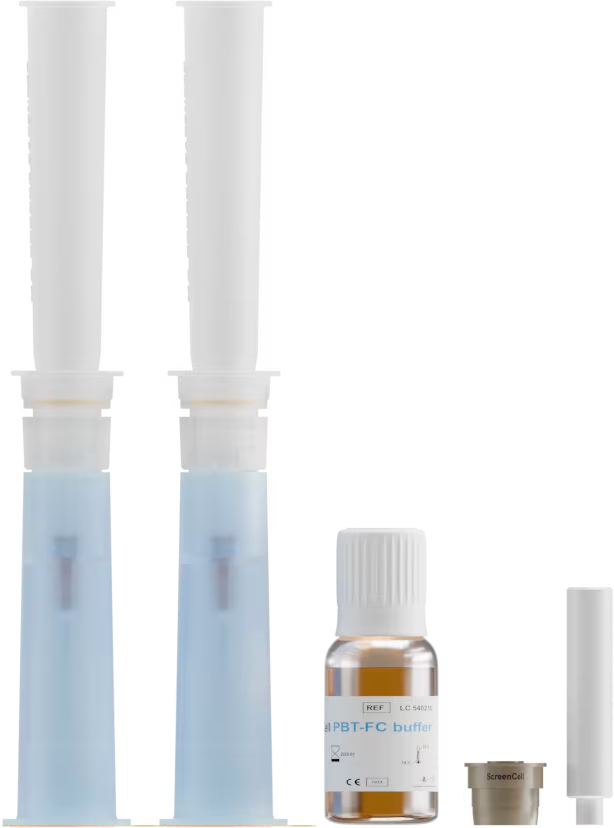
Échantillon de sang
< 3 jours
ou
Cellules tumorales fixées
en suspension ou sur lame
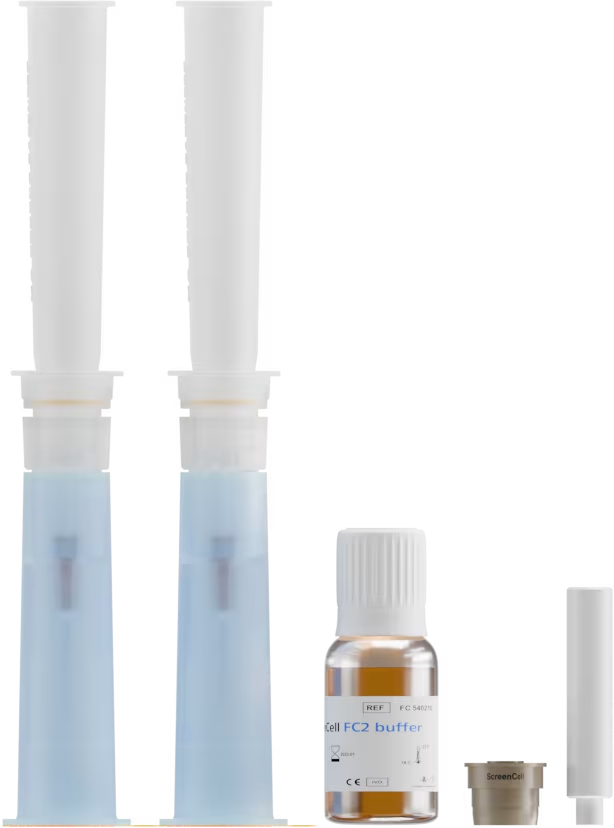
Échantillon de sang
< 4 heures
ou
Cellules tumorales fixées
en suspension ou sur lame

Échantillon de sang
< 4 heures
Cellules tumorales vivantes
sur le support d’isolement

Échantillon de sang
< 3 jours
Cellules tumorales vivantes
sur le support d’isolement

Échantillon de sang
< 4 heures
Cellules tumorales vivantes
sur le support d’isolement
Découvrez plus de 70 articles scientifiques rédigés par nos clients et partenaires.
Vous êtes au bon endroit !
Remplissez les champs ci-dessous et nous nous occupons du reste.
Suivez ScreenCell® sur LinkedIn