Circulating tumor cells are cancer cells that have detached from the primary tumor or metastases and travel through the bloodstream. They play a pivotal role in metastatic dissemination by colonizing distant organs and establishing new tumor lesions.
Cancer cells have the potential to serve as a dynamic and non-invasive biomarker for monitoring solid tumors.
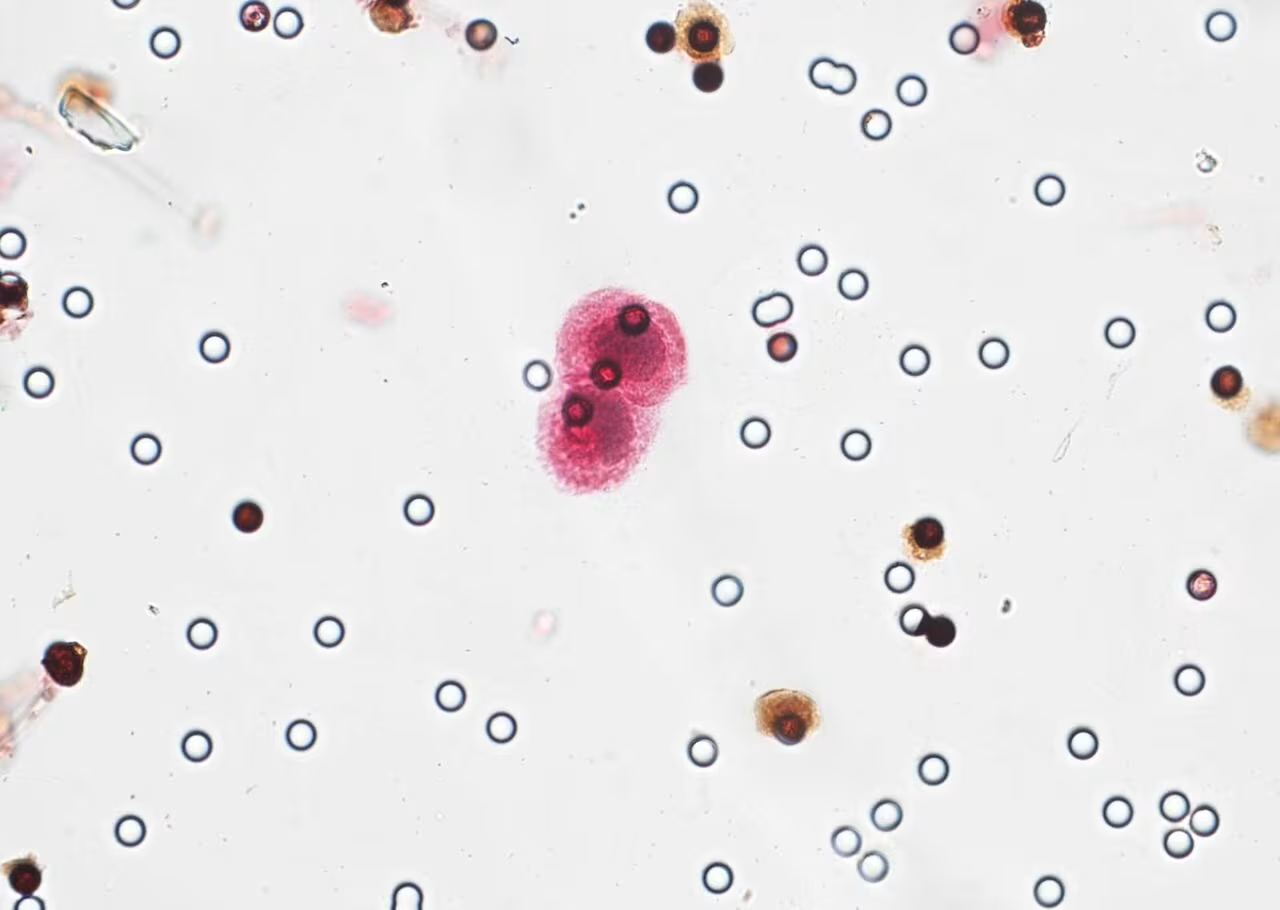
Circulating tumor cells labeled by immunocytochemistry under the microscope

ScreenCell kits provide a simple, effective, and accessible solution for isolating and characterizing CTCs from a single blood sample.
They are designed for versatile use, and can be integrated into both routine clinical practice and basic research projects.
Diagnostic assistance
Evaluation of the effectiveness of the treatment
Cancer monitoring during the remission phase
Early detection of relapses
These cancer cells could be instrumental in the cancer care pathway, offering a non-invasive method for disease monitoring and management.
Our kits empower clinicians to gather crucial insights into tumor progression and treatment response, facilitating personalized care and enhancing therapeutic decision-making throughout the patient journey.
Researchers are paying increasing attention to circulating tumor cells for their role in cancer detection and monitoring.
Here are some key publications in which CTCs were studied using our isolation method.
Circulating cancer cells offer several advantages over traditional biopsies. They are detected through a simple blood test, making the method non-invasive, safer and less painful. In addition, this approach allows for regular and dynamic monitoring of the evolution of the cancer, unlike a tissue biopsy which is often limited to a specific moment.
Circulating cancer cells could play a key role as biomarkers for early diagnosis of cancer and monitoring its progression. Their analysis would allow monitoring the effectiveness of treatments and adjusting them based on the changes observed.
Circulating cancer cells are being studied in solid cancers such as breast, prostate, lung, and colorectal cancer. They may also be relevant for other cancers where the risk of metastasis is high.
No, circulating cancer cells do not replace other methods such as imaging or tissue biopsies.
They are a complementary tool, providing additional information for more precise and personalized care.
Are you interested in establishing a fundamental or clinical research project focused on circulating tumor cells (CTCs) or liquid biopsy ?
Share your project details with us, and we will be delighted to assist you in bringing it to fruition.